Quenching fluids: defects, causes and remedies
Written in the framework of the "Fluids and quenching systems" committee
N.B.: The information contained in this sheet comes from reliable sources. Nevertheless, it is provided without any guarantee, express or implied, of its accuracy.
1. PURPOSE
Update and creation of a dedicated chapter for the book "Tempering fluids".
2. Tempering oils
2.1. Possible faults in the fluid
Oxidation
Oxidation is a radical chain mechanism, initiated by the presence of oxygen in the medium surrounding the quenching oil.
The first phase of the oxidation process consists in the formation of a free radical noted R-, very reactive and with a very short life span, which reacts with oxygen to form a hydroperoxide noted ROOH. This last one being unstable, it generates new free radicals, which induce in their turn new chain reactions which have for effect to accelerate again the oxidation; from where the autocatalytic and exponential character of the reaction of oxidation, making it very difficult to stop.
Table 1 : Chemical reactions involved in the different phases of the oxidation process
The oxidation kinetics is characterized by a more or less long induction period, depending on the nature of the hydrocarbons and the conditions, especially the temperature.
Some trace substances have a very sensitive influence on the duration of the induction period and the oxidation rate. Thus, transition metals catalyze the decomposition of hydroperoxides according to the following mechanism:
Mn+ + ROOH ® M(n+1)+ + RO- + OH-
M(n+1)+ + ROOH ® Mn+ + H+
This applies to most common metals such as chromium, manganese, cobalt and nickel, and especially to iron and copper. Some oxidation products can corrode the metal; in other words, the more the oil oxidizes, the more corrosive it becomes. It then dissolves more metal, which further accelerates the oxidation process.
The oxidation process is accompanied by the transformation of fats into fatty acids (carboxylic acids) and the production of macromolecules, resulting in an increase in the acidity and viscosity of the quenching oil. Aldehydes, ketones, alcohols, esters and carbon dioxide can also be formed.
Table 2 Factors promoting oxidation of quenching oils, consequences and possible remedies
Figure 1: comparison of infrared spectra of a new oil and after an accelerated oxidation of 96h by oxygen bubbling: highlighting the oxidation peak at 1710cm-1 (from Renault document)
Thermal shock - cracking :
The sudden contact of the oil with the parts at high temperatures causes the oil to crack. The long carbon chains of the base oil undergo shearing, leading to the formation of light fractions.
Table 3 Consequences and possible remedies of cracking of quenching oils
Additive consumption :
Two opposite phenomena occur in parallel on the baths in service and modify the characteristics of the oil:
- When new, wetting agents and gas pedals confer a certain drasticity to the quenching oil, the higher their concentration.
On the bath in service, these additives are consumed preferentially by burning or entrainment by the parts. This results in a decrease of their concentration, which has as a consequence a modification of the drasticity curve(less drastic oil).
- Antioxidant additives are acidic compounds, and therefore contribute to the acid number of the new quench oil. The consumption of these antioxidant additives results in a decrease of the acid number during the first weeks of use of a new oil bath. This phenomenon is then compensated by an increase in the acid number due to the oxidation of the oil, which leads to the formation of carboxylic acids. The latter act as wetting agents, thus accelerating the cooling process.
Pollution :
The potential contaminants are of several kinds as summarized in the following table:
Table 4 Origin, consequences and possible remedies for contamination of quenching oils
Some of these solids are partially solubilized, others are finely divided and dispersed, and thus kept in suspension in the oil.
2.2. Detection of the defect
2.2.1. By physico-chemical characterizations
Drasticity:
The drasticity of the quenching oil is measured with a drasticometer.
Table 5 Possible causes of variations in the drasticity of a quenching oil
Color and appearance:
The color of a new oil depends on its degree of refining, its content of aromatic and/or nitrogenous compounds and its additives. The reaction of certain aromatic compounds to oxidation gives very colored products.
Determined by simple visual appreciation or by comparison with a standardized scale, the evolution of color and appearance give an indication of the condition of the quenching oil in service and the possible presence of solid impurities.
Viscosity (mm²/s or cSt) :
Determined by the time of flow by simple gravity of a given volume of liquid through a calibrated capillary viscometer, at a defined temperature (generally 40°C or 100°C), the measurement of viscosity makes it possible to highlight a degradation of the oil.
Table 6 Possible causes of viscosity variations of a quenching oil
The oxidation products, by becoming very polar, can form insoluble products, which can remain in suspension in the oil, or deposit on the installation. This has a strong influence on the evolution of the viscosity, which can pass through a maximum, linked to the thickening due to the insolubles, and then decrease when they are deposited on the walls or in the circuits of the installation.
Acid value and base value (mg(KOH)/g) :
These indices are determined by dosage. Acidity is found mainly in used oils in the form of organic or mineral acids, especially carboxylic acids resulting from the oxidation of hydrocarbons, but new oils can also have an acidic character brought by certain additives such as antioxidants (phenolic compounds, among others).
A reserve of alkalinity, provided by detergents (phenates, salicylates, sulfonates), and to a lesser extent by certain ashless dispersants (succinimides), is necessary to be able to neutralize the acidic compounds that are formed during the use of the oil.
The same oil can have both an acid and a base number, because its acidic and basic constituents are not all able to neutralize each other under normal conditions of use.
The determination of the acid and base indices thus makes it possible, for a new oil, to know its initial properties and in particular its reserve of alkalinity and for an oil in service, to follow its degree of deterioration by the increase of the acid index (oxidation) and the lowering of the base index (consumption of additives).
Table 7 Possible causes of acid number variations in a quenching oil
Table 8 Possible causes of variations in the base index of a quenching oil
Water content (ppm or %):
The percentage of water in the quenching oil is measured by the Karl Fischer method. A water content higher than 500ppm or 0.05% is to be avoided, among other things because it significantly modifies the drasticity of the quenching oil, by increasing the cooling rate.
Figure 2 Influence of the presence of water on the drasticity curve
Flash point (°C) :
The flashpoint is the minimum temperature to which an oil must be heated before it emits enough vapour to form a gaseous mixture with the surrounding air, which ignites under the effect of a flame, but without combustion persisting, hence the term "flash".
The measurement is made under standard conditions, in open vessel according to the Cleveland method or in closed vessel according to the Pensky-Martens method. It is recommended to use the method that is closest to the conditions under which the quenching oil is used in service.
Closed cup flash point values are necessarily lower than open cup flash point values, but no correlation could be established between the two.
Knowing the flash point provides information about the volatility and flammability of the oil.
For safety reasons, it is generally recommended that the flash point of the quenching oil in service be 40-50°C higher than the operating temperature of the oil in the installation in question.
Table 9 Possible causes of flash point variations of a quenching oil
Impurity content (mg/L):
The impurity content is determined gravimetrically, i.e. by filtering a given volume of liquid through a 5µm or 8µm porosity membrane and weighing it.
Table 10 Possible causes of an increase in the gravimetry of a quenching oil
It is also possible to make other analyses from the filter, in order to identify more precisely the nature of the impurities: calcination, infrared by ATR, SEM coupled to the elemental analysis...
Infrared spectrum:
The infrared spectrum, which is acquired in transmission between two KBr plates, allows to quickly visualize the state of the quenching oil and to highlight in particular the phenomena of oxidation, cracking, consumption of additives or pollution, by comparison with the reference spectrum of the new oil.
Table 11 Possible changes in the infrared spectrum of a quenching oil in service compared to new oil
Other methods of characterizing quenching oils
Other methods, not used in monitoring, allow for further characterization of new and in-service quench oils:
o Density: identification of base oils, pollution.
o Viscosity index: effect of a temperature variation on the viscosity of an oil.
o Elemental content by ICP: pollution, wear metals, evolution of the composition of the oil provided that the initial formulation is known.
o Ash content: presence of metallic compounds (additives or contaminants).
o Insoluble pentane (or other solvent): contamination by solids.
o Conradson residue or Ramsbottom residue: propensity to form coke (carbonaceous residue) by pyrolysis.
o Oxidation resistance tests: TOST, Petro-Oxy, DSC under O2, PDSC.
o Etc.
2.2.2. Defects on parts
Note: The defects listed in the table below may also be caused by problems not attributable to the quenching oil.
Table 12 Observable defects on parts and possible causes
3. Aqueous fluids (Quenching polymers)
During its use, the quenching fluid undergoes important stresses such as thermal shocks (cracking) or pollution of various origins. In addition to good thermal shock resistance, a quenching polymer must have good corrosion protection, resistance to foaming and biostability (resistance to micro-organisms) to guarantee the quality of the treated parts, the life of the bath as well as the safety of people and installations.
In order to maintain consistent properties throughout the life of the bath, compliance with bath assembly procedures and, above all, regular monitoring are essential.
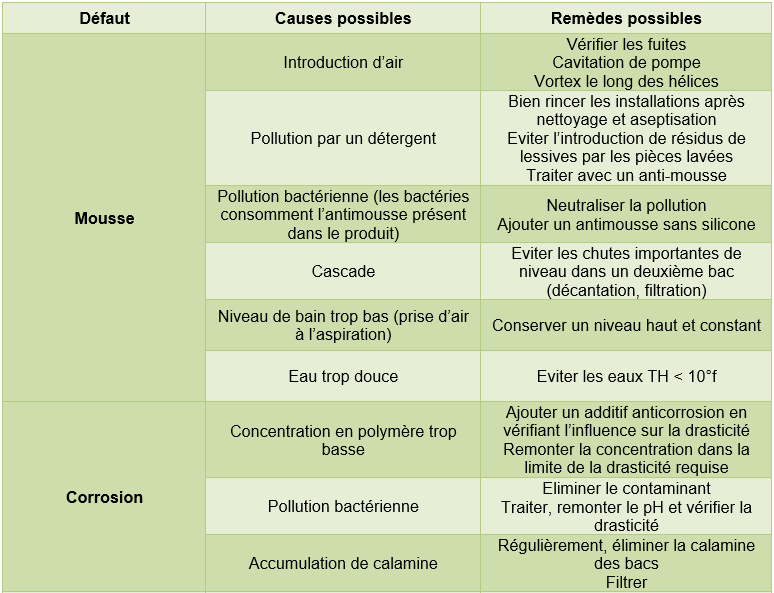
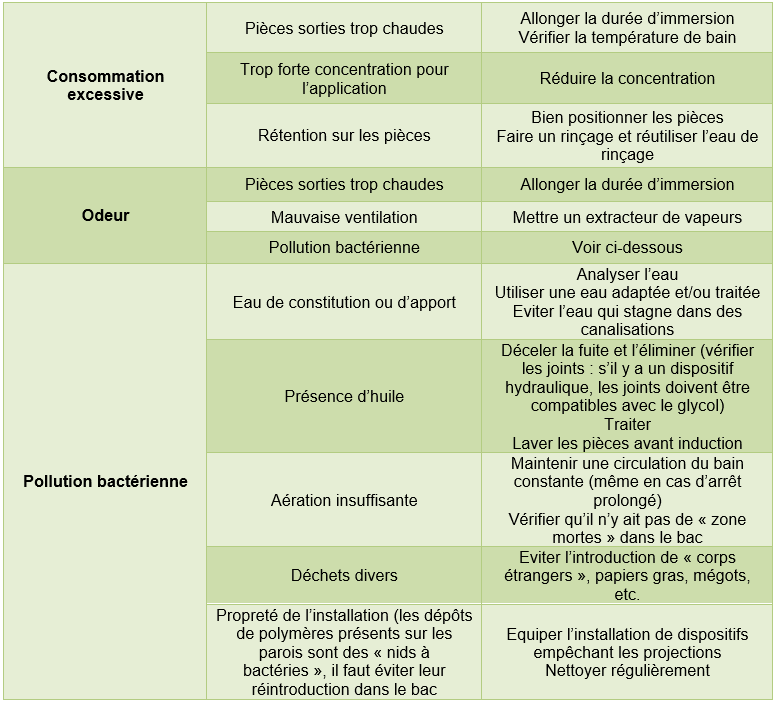
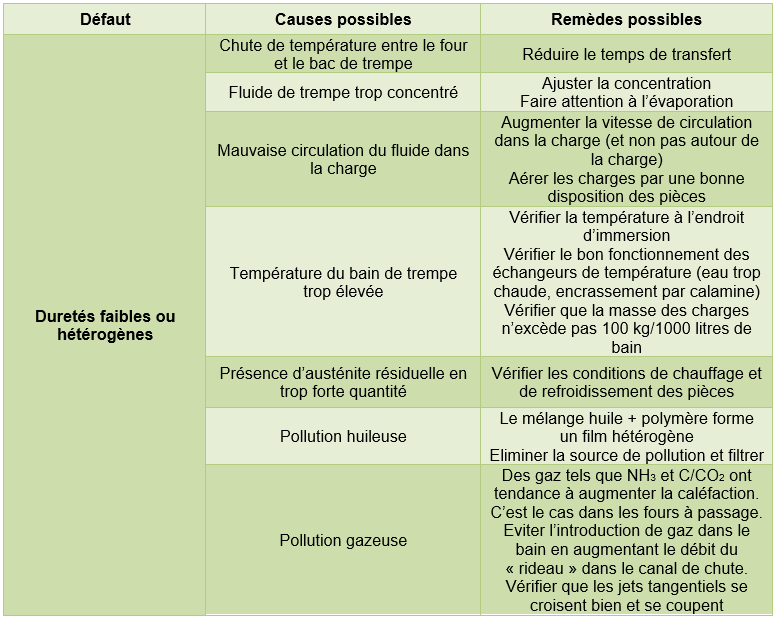
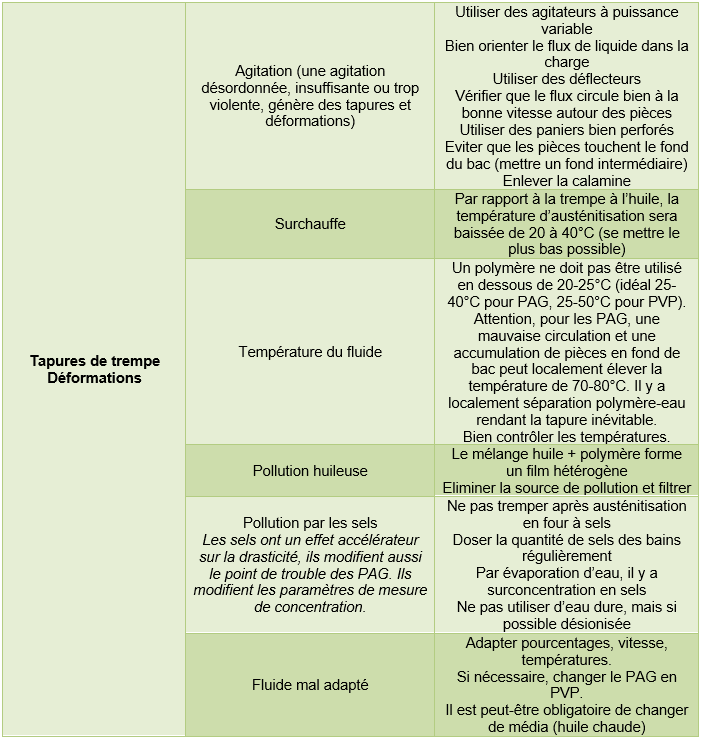
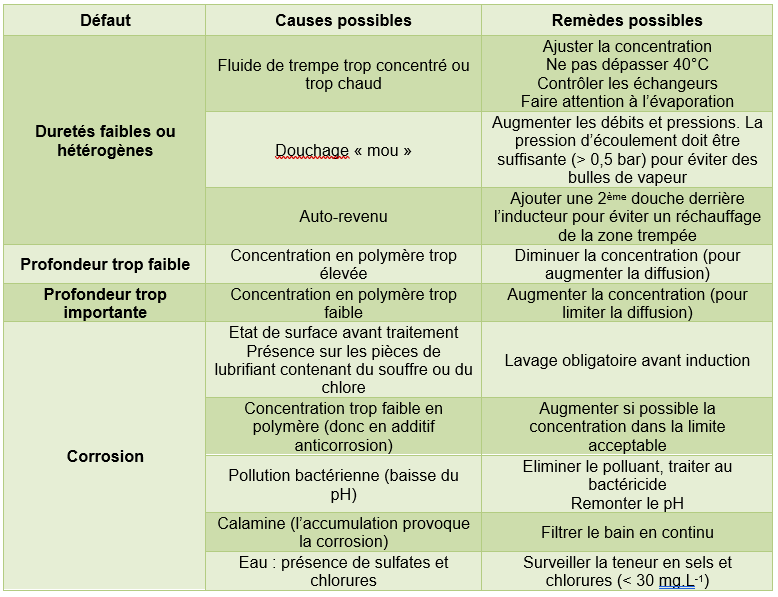
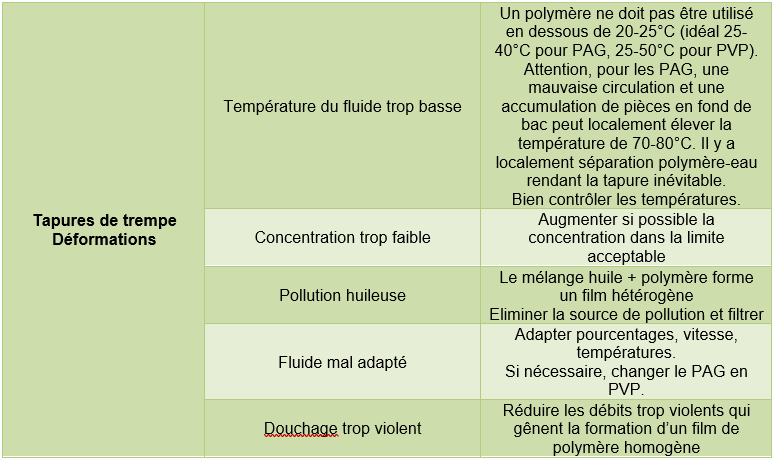
3.1 Possible faults in the fluid
3.2. Detection of the defect
3.2.1 By physico-chemical characterizations
The table below lists for each parameter the causes, consequences and possible remedies of a drift.
Table 14 Causes, consequences and possible remedies for drift in a polymer quench bath
3.2.2. Defects on parts
Note: The defects listed in the tables below may also be caused by problems not attributable to the aqueous fluid.
Tempering in the mass
Surface hardening (induction heating)
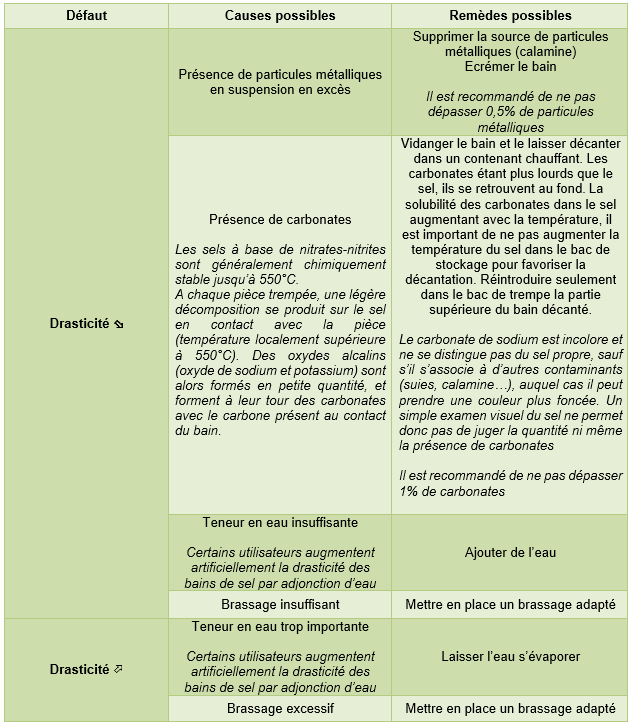
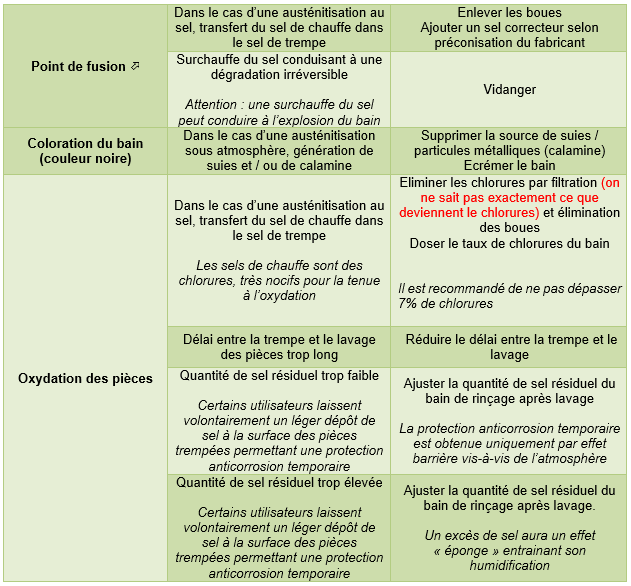
4. Tempering salts
Note: The defects listed in the tables below may also be caused by problems not attributable to the quenching salt. This paragraph does not deal with preheating, tempering (which can however be identical to quenching salts) and austenitizing salts.
Because of their specificity and relative danger, it is strongly recommended to contact the product manufacturer in case of problems.
Quenching salts, provided that the manufacturers' recommendations are strictly followed, are known for their high thermal stability and relative tolerance to contamination. Nevertheless, the following defects and associated remedies can be listed:
4.1. Nitrite / nitrate hardening salts (hardening of low alloy steels)
4.2. Chloride hardening salts (hardening of alloy steels - tool steels)
Table 18 Observable defects on parts and possible causes